75道经典AP化学选择题型(三)
今天继续分享75道经典常考的AP化学选择题三,赶紧来锻炼一下吧!相应的词汇请:AP化学高频词汇。
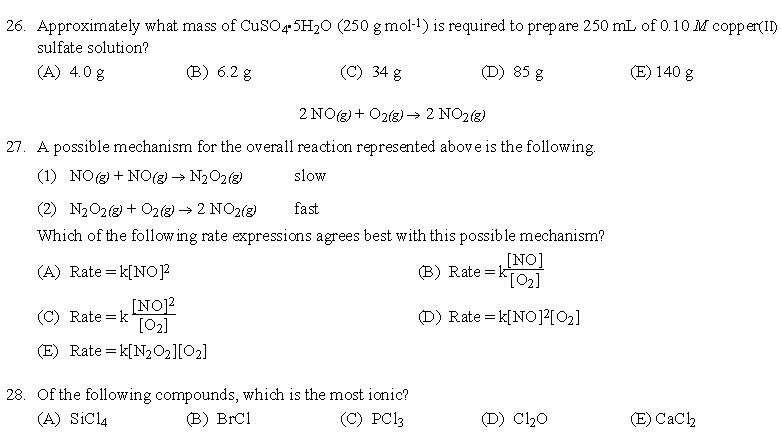
29. The best explanation for the fact that diamond is extremely hard is that diamond crystals
(A) are made up of atoms that are intrinsically hard because of their electronic structures
(B) consist of positive and negative ions that are strongly attracted to each other
(C) are giant molecules in which each atom forms strong covalent bonds with all of its neighboring atoms
(D) are formed under extreme conditions of temperature and pressure
(E) contain orbitals or bands of delocalized electrons that belong not to single atoms but to each crystal as a whole
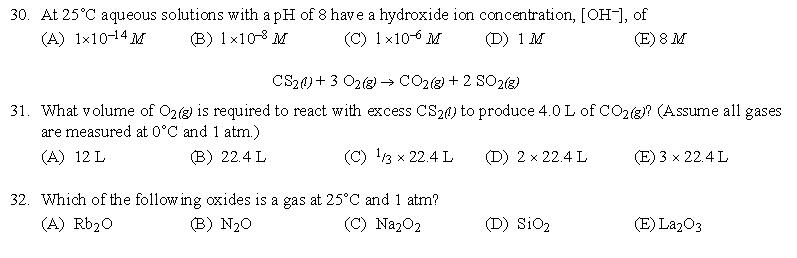
Questions 33-34
The graph below shows the titration curve that results when 100. mL of 0.0250 M acetic acid is titrated with 0.100 M NaOH.

33. Which of the following indicators is the best choice for this titration?
Indicator pH Range of Color Change
(A) Methyl orange 3.2 - 4.4
(B) Methyl red 4.8 - 6.0
(C) Bromothymol blue 6.1 - 7.6
(D) Phenolphthalein 8.2 - 10.0
(E) Alizarin 11.0 - 12.4
34. What part of the curve corresponds to the optimum buffer action for the acetic acid/acetate ion pair?
(A) Point V
(B) Point X
(C) Point Z
(D) Along all of section WY
(E) Along all of section YZ
35. A solution is made by dissolving a nonvolatile solute in a pure solvent. Compared to the pure solvent, the solution
(A) has a higher normal boiling point
(B) has a higher vapor pressure
(C) has the same vapor pressure
(D) has a higher freezing point
(E) is more nearly ideal

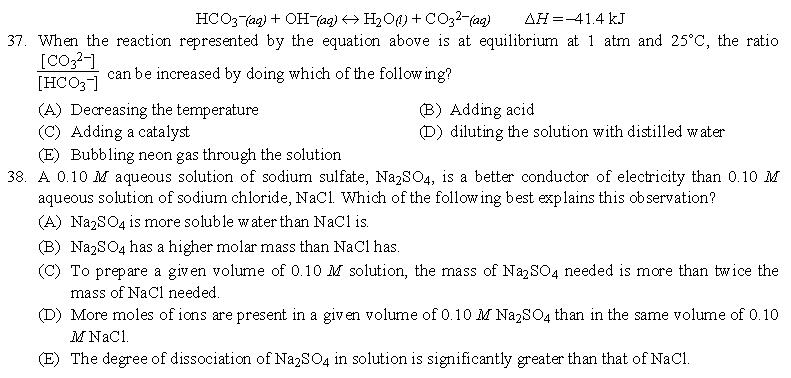
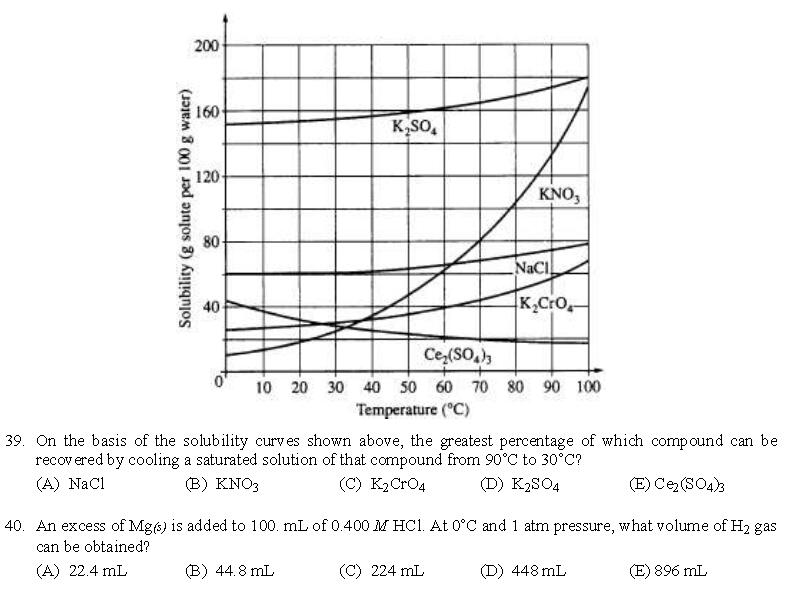
答案:26、B | 27、A | 28、E | 29、C | 30、C | 31、A | 32、B | 33、D | 34、A | 35、A | 36、B | 37、A | 38、D | 39、B | 40、D









