75道经典AP化学选择题型(二)
今天继续分享75道经典常考的AP化学选择题2,赶紧来锻炼一下吧!答案在文章结尾。
Part B
Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the corresponding oval on the answer sheet.
Questions 15-16 relate to the graph below. The graph shows the temperature of a pure substance as it is heated at a constant rate in an open vessel at 1.0 atm pressure. The substance changes from the solid to the liquid to the gas phase.
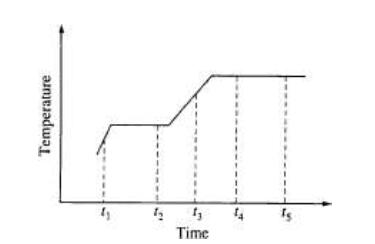
15. The substance is at its normal freezing point at time
(A) t1 (B) t2 (C) t3 (D) t4 (E) t5
16. Which of the following best describes what happens to the substance between t4 and t5?
(A) The molecules are leaving the liquid phase.
(B) The solid and liquid phases coexist in equilibrium.
(C) The vapor pressure of the substance is decreasing.
(D) The average intermolecular distance is decreasing.
(E) The temperature of the substance is increasing.
17. In which of the following groups are the three species isoelectronic; i.e., have the same number of electrons?
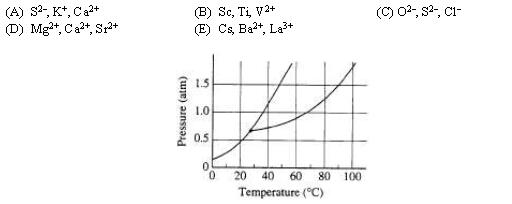
18. The phase diagram for the pure substance X is shown above. The temperature of a sample of pure solid X is slowly raised from 10˚C to 100˚C at a constant pressure of 0.5 atm. What is the expected behavior of the substance?
(A) It first melts to a liquid and then boils at about 70˚C.
(B) It first melts to a liquid and then boils at about 30˚C.
(C) It melts to a liquid at a temperature of about 20˚C and remains a liquid until the temperature is greater than 100˚C.
(D) It sublimes to vapor at an equilibrium temperature of about 20˚ C.
(E) It remains a solid until the temperature is greater than 100˚C.

答案:15B 16A 17A 18D 19E 20E 21B 22C 23D 24C 25A
更多AP化学真题及考点,请关注:https://sh.xhd.cn/apchemistry/
新航道上海AP课程培训中心,是新航道集团和美国Kaplan教育集团共建设,美国大学理事会在中国授权的AP嵌入式课程学习中心,AP Code:694387。
我们开设有多重AP课程,感兴趣的同学还可以在线咨询哦









