SAT2 化学85道题考点分布详解
5月SAT考试在即,新航道SAT给大家带来了SAT2化学考题知识点分析,更多SAT真题下载,请:https://sh.xhd.cn/sat/baokaochangshi/
一. 关于 SAT 化学考试的格式和注意事项
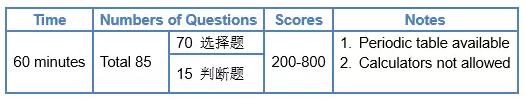
二. 85道题在大纲所考察各个知识点板块中的具体分布
Structure of Matter
Contents
Atomic Structure, including experimental evidence of atomic structure, quantum numbersand energy levels (orbitals), electron configurations,periodic trends,nuclear chemistry
Molecular Structure, including Lewis structures, three-dimensional molecular shapes,polarity
Bonding, including ionic,covalent and metallic bonds;relationships of bonding to properties and structures; intermolecular forces such as hydrogen bonding,dipole-dipole forces, dispersion (London) forces
No. of Questions
21-22 Questions
2
States of Matter
Contents
Gases, including the kinetic molecular theory, gas law relationships, molar volumes, density and stoichiometry
Liquids and Solids, including intermolecular forces in liquids and solids, types of solids,phase changes and phase diagrams
Solutions, including molarity and percent by mass concentrations, solution preparation and stoichiometry,factors affecting solubility of solids, liquids and gases,qualitative aspects of colligative properties
No. of Questions
13 – 14 Questions
3
Reaction Types
Contents
Acids and Bases, including Brosted-Lowry theory, strong and weak acids and bases, pH, titrations,indicators
Oxidation-Reduction, including recognition of oxidation-reduction reactions, combustion, oxidation numbers, use of activity series
Electrochemistry
Precipitation, including basic solubility rules
No. of Questions
11 – 12 questions
4
Stoichiometry
Contents
Mole Concept, including molar mass, Avogadro’s number, empirical and molecular formulas, Chemical Equations, including the balancing of equations, stoichiometric calculations,percent yield and limiting reactants
No. of Questions
11 – 12 questions
5
Equilibrium and Reaction Rates
Contents
Including factors affecting position of equilibrium (Le Chatelier’s principle)in gaseous and aqueous systems,equilibrium constants, and equilibrium expressions, Factors affecting reaction rates, potential energy diagrams, activation energies
No. of Questions
4 – 5 questions
6
Thermochemistry
Contents
Including conservation of energy, calorimetry and specific heats, enthalpy(heat) changes associated with phase changes and chemical reactions,heating and cooling curves,entropy
No. of Questions
5 – 6 questions
7
Descriptive Chemistry
Contents
ncluding common elements,nomenclature of ions and compounds, periodic trends inchemical and physical properties of the elements, reactivity of elements and prediction of products of chemical reactions, examples of simple organic compounds and compounds of environmental concern
No. of Questions
10 – 11 questions
8
Laboratory Knowledge
Contents
Including knowledge of laboratory equipment, measurements, procedures, observations, safety,calculations, data analysis, interpretation of graphical data, drawing conclusions from observations and data
No. of Questions
6 – 7 questions
三. 在SAT化学考试中,试题可以分为三类,对应Collegeboard对三类能力的要求

其中,Fundamental concepts and knowledges 是对基本化学概念和原理的考察,相当于要求考生把这些化学定理、定律重述一遍。
Applying of knowledge 是用化学理论对一些不熟悉的实际问题进行分析,得出定性结论或做简单的定量计算。
Synthesis of knowledge 是运用多个化学知识点,由题中给出的数据和图标归纳、提炼出答案。
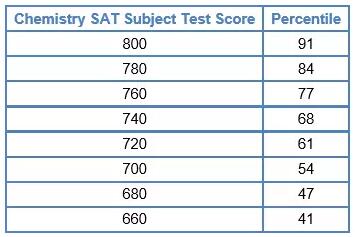
五. 分数转换与备考建议
下面的表格是SAT化学分值所对应的百分比。
例如,800分表示达到了top 9%的水平。
Collegeboard建议准备SAT化学考试的同学上一年大学预科级别的化学入门课,有一定的化学实验基础,学过一年的代数课。
SAT化学考试所考察的内容多且杂,包括了所有化学分支学科(inorganic,organic, analytic, physical, structural chemistry)。
如果想在短时间内掌握SAT化学考试大纲要求的所有知识点,能选择一本exam guide,多做练习题。
其中,一定重视Collegeboard的官网习题。SAT化学考题虽然不会重复使用,但是所考察的内容和题型是不变的。
只要准备充分,拿到SAT化学满分是完全可以实现的。








